Organic Chemistry Basics: How To Hack The 6 Pillars of Organic Chemistry
In previous blog posts I've talked a lot about organic chemistry basics.
Why? Because organic chemistry is a subject that rewards you for knowing the basics and knowing them well.
That's where your white coat is.
That's how you're going to avoid disappointing your family.
Mastering the basics of ochem is as close as you're gonna get to organic chemistry hacks.
Many people refer to these basics as organic chemistry pillars. This comes from the article written by Joseph Mullins of Le Moyne College in Syracuse, NY. (It's behind a paywall, but you should be able to access it with your university credentials - https://pubs.acs.org/doi/abs/10.1021/ed085p83)
He describes the 6 pillars as the following:
- Electronegativity
- Polar Covalent Bonding
- Steric Effects
- Inductive Effects
- Resonance
- Aromaticity
In this post, I'm going to touch on each pillar as described by Dr. Mullins and how you can hack it to force ochem to give you the A that you want.
Organic Chemistry Pillar #1 - Electronegativity
What is Electronegativity?
Electronegativity is the property of an atom to pull electron pairs toward itself.
You can think about electronegativity as the ability of an atom to steal the covers from the other atoms in the bed with it at night. Some atoms play nice and make sure that you have plenty of cover through the night.
Some atoms aren't as considerate and will snatch the most amount of cover away from you in the middle of the night, leaving you cold and shivering.
They'll also take up as much of the bed as possible.
Anyhow, each atom has a specific electronegativity. This determines how it affects other atoms around it, and ultimately how the rest of the molecule behaves.
When it comes to the periodic table, electronegativity tends to increase as you go up and across the table as shown below as indicated by the numbers under each element below.

Why is Electronegativity Important?
Consider electronegativity as it relates to organic reactions.
Specifically, let's look at the basicity of some anions that have atoms in the same period.

Dr. Mullins uses this example to drive home some points about electronegativity.
Carbon, nitrogen, oxygen, and fluorine are all in the same period. As you go from left to right in that period the electronegativity increases, but the basicity/nucleophilicity decreases. The opposite happens as you go from right to left in this period.
This trend is important to understand in acid/base chemistry and organic reaction mechanisms. Knowing this trend can help you recognize what side of acid/base equilibria is favored and what atom will be attacked by a nucleophile.
Using Electronegativity to Hack Organic Chemistry
Acids and Bases
As stated above, electronegativity can help you determine which acid/base pair is favored. Let's look at an example below.

In the above equation, the left side has a very strong base in the carbanion and a weak acid in water. How do you know it is weak?
Water is the conjugate acid of hydroxide, which is considered a strong base. If you have a strong base, then the conjugate acid must be weak and vice versa.
After the neutralization reaction occurs, you're left with pentane, which is a very weak acid and hydroxide, which is a moderately strong base. If you were to solely look at the chart of electronegativities, you could instantly recognize that the right side should be favored because the methyl carbanion (in red) is the strongest base/nucleophile of the period.
Equilibrium favors the weaker acid and base pair
Although it is not the sole determining factor, the atom type is important, especially when pKa's are unavailable. This is qualitative acid/base strength analysis which uses a very handy acronym known as ARIO
ARIO stands for:
- Type of Atom holding the charge
- Resonance
- Inductive Effects
- Orbital type holding the charge
Electronegativity falls under the type of atom holding the charge. The more electronegative an atom is the easier it can stabilize a negative charge since it wants to snatch covers, I mean electrons.
Now let's verify our guesstimate using pKa's.

The pKa measures fit right in line with our pick of the right side being favored.
If you know the trends in electronegativity, then you can quickly get a clue to which side of the equation will be favored.
Predicting Nucleophilic Attack
When you look at the names nucleophile and electrophile let's break down what the words mean.
Nucleophile = Likes nucleus
If the nucleus is positively charged, then something has to be negatively charged to like it. Remember that like charges repel each other.
This must mean that the nucleophile has an abundance of electrons to share
Electrophile = Likes electrons
If something likes electrons, which are negative, then that must mean this thing has a positive charge.
How does this relate to electronegativity? Gud Kweschun. Let's look at an example below.

When looking at bromopropane, the bromine is more electronegative than the carbon it is next to. All of the other carbons are next to other carbons and hydrogens, which have a similar electronegativity.
This means that there is a partial positive charge created from bromine snatching the covers. Remember what we said about electrophiles.
They have to be positively charged.
In this case, the partial positive charge created by the electronegativity difference between carbon and bromine is enough for carbon to be the electrophile.
It is then attacked by the hydroxide nucleophile to create propanol.
When there is a large enough electronegativity difference between 2 atoms, the atom that is the least electronegative will likely be your electrophile. (Quick thing to note, hydrogen will NEVER be an electrophile outside of acid/base chemistry.)
Of course, these are general guidelines for using electronegativity. Some examples will not go strictly by this rule as there are other factors to consider (remember ARIO).
Yet, engraining these organic chemistry hacks related to electronegativity into your mind will help you quickly identify favored acid/base pairs and which atom will likely be attacked by a nucleophile, 2 key subjects in organic chemistry.
Now that you know how to hack organic chemistry using electronegativity, read ahead to the next pillar to hack, polar covalent bonds.
Organic Chemistry Pillar #2 - Polar Covalent Bonds
What are Polar Covalent Bonds?
Polar covalent bonds are a type of covalent bond where the majority of the electrons in the bond spend more time with one atom or set of atoms than the other.
In other words, the greedy atoms get to sleep under a warm electric blank throughout the night while the other atoms sleep out in the cold. What does this remind you of?
Electronegativity, which we just talked about above.
Polar covalent bonds are a direct result of differences in electronegativity as shown in the bromopropane example above. Another example of a common organic substance that would have polar covalent bonds is methylene chloride shown below.

When you have a polar covalent bond, the difference in electronegativity creates what's known as a dipole moment. Dipole means two poles, so that must mean that this difference creates a moment when there are two detectable poles, positive and negative.
This is why atoms in a polar covalent bond are shown with a partial positive and a partial negative as shown above in the bromopropane example.
Why are Polar Covalent Bonds Important?
Polar covalent bonds are important in a similar way to electronegativity.
This is because these bonds exist due to differences in electronegativity. Knowing what parts of your molecule are electron rich and electron poor will help you quickly predict which parts will be reactive and who their partners are.
Reactions at the core are a series of pluses and minuses trying to find dance partners in each other. Think about it.
- Nucleophiles (-) attack electrophiles (+)
- Bronsted-Lowry Bases (-) accept protons from Bronsted-Lowry Acids (+)
- Lewis Bases (-) donate electron pairs to Lewis Acids (+)
One of the yhuuuuge factors these reactions are driven by is polar covalent bonds. Learning how to spot them easily will help you find many important reaction patterns in organic chemistry.
Another thing to consider with polar covalent bonds is how these molecules interact with each other on the molecular level outside of the reactions above.
Molecules that have polar covalent bonds have a type of intermolecular force called dipole-dipole interactions. This is the 2nd strongest intermolecular force available for covalently bonded molecules.
The strength of this force affects how molecules behave on a structural level.
It also affects other properties such as:
- boiling point
- melting point
- vapor pressure
- solubility in water
- solubility in non-polar solvents
These properties will be discussed ahead.
Using Polar Covalent Bonds to Hack Organic Chemistry
Predicting Physical Properties Affected By Intermolecular Forces
Polar covalent bonds can also be used to predict reactions, but we covered that in the electronegativity section.
If you want to see that then pop back up above one pillar. Here we'll focus on how polar covalent bonds affect physical properties. Let's talk about measurable physical properties first.
Consider the 3 molecules below:

From left to right, you have a simple alkane, a haloalkane, and alcohol. The first thing that immediately jumps off of the page is how drastically different the boiling points are even though the structures are similar.
The only thing that changes is the functional group on the carbon.
So what's happening here? Changes in intermolecular force. 
The molecule that has the weakest intermolecular force also has the lowest boiling point. The molecule that has the strongest intermolecular force also has the highest boiling point.
Ethanol's ability to hydrogen bond makes it have the highest boiling point of these 3 compounds that are similar in structure.
The key takeaway here is that polar covalent bonds result in stronger intermolecular forces that affect physical properties. In general, as intermolecular force strength increases, the following also increase and vice versa:
- Melting Point
- Boiling Point
- Water Solubility
As intermolecular force strength increases, the following decrease and vice versa:
- Vapor Pressure
- Non-polar Solvent Solubility
This general observation paired with being able to predict reactions will tell you a lot about how molecules behave and how reactions will proceed.
The next ochem pillar to hack is steric effects and you can find that below!
Organic Chemistry Pillar #3 - Steric Effects
What are Steric Effects?
Steric effects are non-bonding interactions that are affected by the space that atoms and molecules take up.
Even though you write molecules and atoms flat on paper, they still take up space in real life. They are still 3D objects.
Because the molecules take up some amount of space, there are energy changes when molecules interact with each other.
Imagine that you are out and about with your friends. Everyone is having a good time. Drinks are flowing, music is pumping, everyone is enjoying life.
Out of the corner of your eye, you see someone that catches your interest. For the guys, you see the hottest woman in the spot giving you the eye to come over and talk to her.
For the women, you see the stud in the room take a glimpse at you and he begins to walk over. You feel the build-up of tension as you approach them or they approach you.
Guys, you get right into this woman's space and out of nowhere, one of her girlfriends appears and steps right in between you two.
Women, he gets 3 steps away from you and begins to open his mouth when his girlfriend drops in and grabs him by the arm to drag him away.
In an instant, your potential connection is ruined. That's what can happen with steric effects.
Why are Steric Effects Important?
Steric effects are important because they change how molecules interact with each other and whether or not reactions can proceed.
If you have a large steric effect caused by large groups at a reactive center, it's unlikely that the reaction will go forward. The size of the groups attached to the electrophile block the approach of the nucleophile. See an example of this below with substitution reactions.

Steric effects also influence the shape of molecules. Remember that according to VSEPR (Valence Shell Electron Pair Repulsion) theory, atoms want to be in the shape that gets electrons the farthest apart from each other. The electron repulsion in the atoms will sometimes favor one conformation over another like in the example below.

Anti is the generally preferred conformation for these alkanes. This is even more true for butane because of the steric crowding that happens at the terminal methyl hydrogens in the eclipsed conformation.
Using Steric Effects to Hack Organic Chemistry
As explained above, steric effects influence substitution and elimination reactions and molecular conformations.
Steric effects are discussed in great detail with substitution and elimination reactions because they have a great influence over products and intermediates. They also influence molecules to prefer certain shapes over others.
When thinking about steric effects here are some things you want to ask yourself:
- If you replace groups, does that reduce or increase steric effects
- If you add a specific group, more groups, or less groups, how does the reaction change
- If you physically rotate a molecule, are the groups attached likely to run into each other
As you get farther into your ochem studies, you will also see that those steric effects have a great influence over other small, but important factors like how molecules dissolve in solution.
Next, let's talk about the fourth pillar, inductive effects below!
Organic Chemistry Pillar #4 - Inductive Effects
What are Inductive Effects?
Inductive effects are a result of sigma bond polarization due to either electron withdrawing effects or electron donating effects.
As long as atoms are connected via sigma bond, they can feel inductive effects. Think about making a "telephone" with a string between two cups.
The cups are the atoms.
The string is the sigma bond.
Speaking is an electron donating effect.
Listening is an electron withdrawing effect.
Why are Inductive Effects Important?
Inductive effects are important because that can stabilize or destabilize atoms in a molecule.
The same is true for ionic intermediates. Remember that molecular stability influences how or if molecules will react.
Depending on the type of inductive effective, they can change:
- nucleophilicity
- electrophilicity
- acidity
- basicity
Keep these things in mind when you are trying to determine how a reaction will go forward or what intermediates are produced.
Using Inductive Effects to Hack Organic Chemistry
Acids and Bases
Inductive effects can be used to change the acidity or basicity of a molecule. Consider the following example below involving acetic acid.

Notice that as you add more chlorine molecules to acetic acid, the pKa continues to drop, meaning it's becoming more acidic.
So what's happening here? The inductive effect of the chlorine atoms is pulling electron density away from the carboxylic acid.
So why is this making it more acidic? Think about the conjugate bases of each.

Remember that oxygen is also very electronegative. It doesn't like to share its covers if it doesn't have to. Since there is such a short trek bond wise from chlorine to oxygen, the inductive pull is felt heavily.
When you get up to three chlorine atoms, there's a full-blown tug-o-war between chlorine and oxygen for electrons. If oxygen is gonna win, it has to get rid of some dead weight in a hurry.
Hydrogen, you are the weakest link. Goodbye.
If oxygen has to give up more electron density through it's bonds, then it will want to let go of the weakest bond possible. Since there is already a large electronegativity difference between oxygen and hydrogen, it is the first to go, making it more acidic.
The key takeaway here is that the more electron withdrawing groups you have, the more acidic your molecule is. The opposite is true with bases. They become less basic because there is less electron density available.
If you add electron donating groups to an acid, it becomes less acidic. A base becomes more basic.
Nucleophiles and Electrophiles
Let's consider addition reactions for a second. Specifically, hydrohalogenation.

The important thing to note above is the generation of a carbocation intermediate. Take a moment to think about what would make a carbocation more stable or less stable.
If you have electron withdrawing groups near the carbocation, they will destabilize the charge, making it more reactive.
If you have electron donating groups near the carbocation, they will stabilize the charge, making it less reactive.
When things are more reactive, they tend to react with anything they can, creating unintended products.
The opposite is true when things are less reactive. Keep in mind here that you want the carbocation to be stabilized enough so that the nucleophile can attack it.
Consider the following example:

According to the regular reaction mechanism, the top product should be the major product. However, it's not.
And it's because of inductive effects. The carbocation created initially is secondary. However, there is the opportunity for it to be tertiary if a hydrogen (green) shifts to the secondary position.
This is known as a hydride shift.
The hydrogen swaps places with the carbocation because there is more donated electron density in that tertiary position than at the secondary position. This stabilizes the carbocation better so that the halogen attack can take place easier.
The key takeaway here is that whenever you have a reaction that produces ionic intermediates such as carbocations, look around the ion to see what inductive effects are nearby. This will influence many reaction intermediates and in some instances give you unexpected products.
The next pillar of organic chemistry that we will look at is resonance below.
Organic Chemistry Pillar #5 - Resonance
What is Resonance?
Resonance is the ability of a molecule to delocalize (spread out) electrons through pi bonds.

The different molecular representations of this delocalization are called resonance contributors. Resonance stabilization can only happen with molecules that have pi bonds.
Resonance contributors are only suggested depictions of what is happening with the electron density through curved arrow formalism. The true resonance form of the allyl carbocation is shown below.
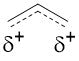
Why is Resonance Important?
Since resonance spreads electron density over space versus condensing it in one spot, this means that resonance makes molecules have lower overall energy.
This means that the molecule is much more stable with resonance than without.
Drawing resonance contributors can also explain why molecules react in a way that is not readily seen on paper.
Using Resonance to Hack Organic Chemistry
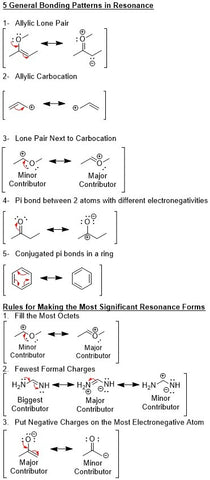
Below are some very straightforward ways to writing resonance structures.
This includes the 5 general bonding patterns in resonance and 3 rules to use to write the most correct resonance structures available.
These graphics come from the hack organic 1 bundle that you can find here.
Below is the last pillar of organic chemistry to hack, aromaticity.
Organic Chemistry Pillar #6 - Aromaticity
What is Aromaticity?
Aromaticity is an unusual state of stability that happens when a molecule meets the conditions below:
- cyclic -- the molecule must be a cyclic polyene;
- planar -- the geometry of the cyclic part must be planar;
- each atom in the cyclic system must have a p orbital perpendicular to the ring;
- the cyclic system has 4n+2 pi electrons.
Aromatic molecules are fairly unreactive due to the fact that this next work of electrons has a high degree of electron delocalization. The model compound that is used to describe aromaticity is benzene

Why is Aromaticity Important?
Aromaticity is important for many of the same reasons that resonance is important.
In fact, aromaticity can be thought of like a supercharged form of resonance. Aromatic compounds are very low in energy. Because of this, they are relatively inert except for specific conditions.
Reactions that would normally affect unsaturated hydrocarbons like alkenes don't affect aromatic compounds. This allows you to incorporate aromatic structures in synthesis without fear of them being transformed by common reactions conditions.
Aromaticity can also affect nearby functional groups. Let's examine the example below.
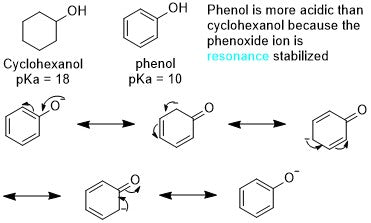
If you have a pi system that extends beyond the aromatic ring like phenol does, any charges that are created can be delocalized within the ring.
This makes the resulting ion much more stable. This increases the likelihood of the alcohol losing its proton.
Using Aromaticity to Hack Organic Chemistry
To be honest, there's no hack to using aromaticity.
Just recognize that it is essentially a subset of resonance that has a special set of rules. You can apply many of the things that you know about resonance to aromaticity as it relates to inductive effects and nearby functional groups.
Conclusion
These six pillars of organic chemistry are very important to understand if you want to have success in the course.
Ingraining these organic chemistry basics into your thought process will help you recognize patterns that keep you from having to remember individual steps and reactions.
This is as close to organic chemistry hacks that you will get. Slam dunk the organic chemistry basics so that you can prosper in this class and get your white coat.
Check out related articles on mastering organic chemistry basics below.
Organic Chemistry Basics: How To Study For Exams Without Wasting Time
Organic Chemistry Class: The Ultimate Guide to Surviving Summer Organic Chemistry
Organic Chemistry Basics: Why Long Study Hours Doesn't Equal Organic Chemistry Success
PS: all graphics except the electronegativity chart come from the hack organic 1 bundle listed here: https://hackorganicchemistry.com/collections/frontpage/products/hack-organic-1-bundle-cheat-sheet-and-practice-tests
